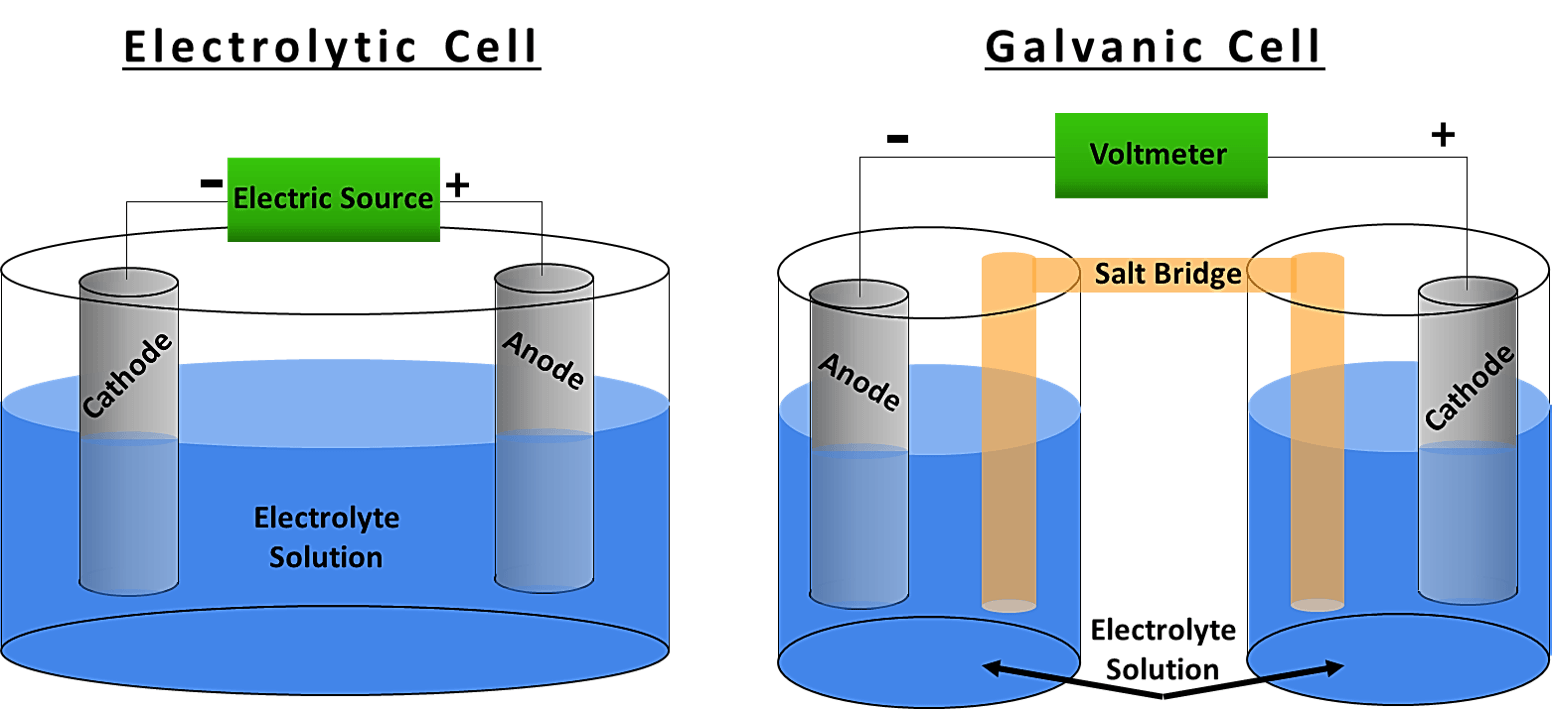
Difference Between Galvanic And Electrolytic Cell . The main differences are outlined below: Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. A galvanic cell converts chemical energy to electrical. Web what's the difference between a galvanic cell and an electrolytic cell? Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web there are two types of electrochemical cells: Web galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. Web however, there are also striking differences between the two cells.
from diagramlibrarypern.z21.web.core.windows.net
Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Web what's the difference between a galvanic cell and an electrolytic cell? The main differences are outlined below: Web there are two types of electrochemical cells: Web galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. A galvanic cell converts chemical energy to electrical. Web however, there are also striking differences between the two cells. Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the.
Cathode In Electrochemical Cell
Difference Between Galvanic And Electrolytic Cell A galvanic cell converts chemical energy to electrical. Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web what's the difference between a galvanic cell and an electrolytic cell? The main differences are outlined below: A galvanic cell converts chemical energy to electrical. Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Web however, there are also striking differences between the two cells. Web there are two types of electrochemical cells: Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Web galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content Difference Between Galvanic And Electrolytic Cell Web there are two types of electrochemical cells: Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Web however, there are also striking differences between the two cells. Web galvanic cells. Difference Between Galvanic And Electrolytic Cell.
From pediaa.com
Difference Between Galvanic and Electrolytic Cell Definition, How Difference Between Galvanic And Electrolytic Cell Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. The main differences are outlined below: Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. Web however,. Difference Between Galvanic And Electrolytic Cell.
From thechemistrynotes.com
Difference and Similarity Galvanic vs Electrolytic Cell Difference Between Galvanic And Electrolytic Cell Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. The main differences are outlined below: Web however, there are also striking differences between the two cells. A galvanic cell converts chemical energy to electrical. Web galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. Web. Difference Between Galvanic And Electrolytic Cell.
From pipingtechs.com
Can You Put Galvanized To Stainless Steel ? Piping Technology System Difference Between Galvanic And Electrolytic Cell The main differences are outlined below: Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Web galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. A galvanic cell converts chemical energy to electrical. Web there are two types of electrochemical cells: Web however, there are. Difference Between Galvanic And Electrolytic Cell.
From circuitdelgerf1.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis Difference Between Galvanic And Electrolytic Cell A galvanic cell converts chemical energy to electrical. Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Web there are two types of electrochemical cells: Web what's the difference between a. Difference Between Galvanic And Electrolytic Cell.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells Difference Between Galvanic And Electrolytic Cell Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. A galvanic cell converts chemical energy to electrical. Web galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. Web what's the difference between a galvanic cell and an electrolytic cell? Web a galvanic (voltaic). Difference Between Galvanic And Electrolytic Cell.
From sciencestruck.com
Similarities and Differences Between Voltaic Cells and Electrolytic Cells Difference Between Galvanic And Electrolytic Cell The main differences are outlined below: A galvanic cell converts chemical energy to electrical. Web galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. Web what's the difference between a galvanic cell and an electrolytic cell? Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web this. Difference Between Galvanic And Electrolytic Cell.
From saylordotorg.github.io
Describing Electrochemical Cells Difference Between Galvanic And Electrolytic Cell Web there are two types of electrochemical cells: Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Web what's the difference between a galvanic cell and an electrolytic cell? The main differences are outlined below: Web galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require. Difference Between Galvanic And Electrolytic Cell.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Difference Between Galvanic And Electrolytic Cell Web there are two types of electrochemical cells: Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. The main differences are outlined below: Web what's the difference between a galvanic cell and an. Difference Between Galvanic And Electrolytic Cell.
From www.youtube.com
Galvanic Cell Vs Electrolytic Cell differences YouTube Difference Between Galvanic And Electrolytic Cell Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. The main differences are outlined below: Web however, there are also striking differences between the two cells. Web what's the difference between a galvanic. Difference Between Galvanic And Electrolytic Cell.
From schoolbag.info
Electrochemistry Review the Knowledge You Need to Score High 5 Difference Between Galvanic And Electrolytic Cell The main differences are outlined below: A galvanic cell converts chemical energy to electrical. Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Web what's the difference between a galvanic cell and an electrolytic cell? Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the.. Difference Between Galvanic And Electrolytic Cell.
From psiberg.com
Galvanic vs. Electrolytic Cell The Two Types of Electrochemical Cells Difference Between Galvanic And Electrolytic Cell Web what's the difference between a galvanic cell and an electrolytic cell? A galvanic cell converts chemical energy to electrical. The main differences are outlined below: Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Web this article explains the key differences between galvanic cell and electrolytic cell on the basis. Difference Between Galvanic And Electrolytic Cell.
From www.vrogue.co
What Is The Difference Between Galvanic Cell And Elec vrogue.co Difference Between Galvanic And Electrolytic Cell Web galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. The main differences are outlined below: Web what's the difference between a galvanic cell and an electrolytic cell? A galvanic cell converts chemical energy to electrical. Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web there. Difference Between Galvanic And Electrolytic Cell.
From mrselliott.wordpress.com
Electrochemistry, featuring electrolysis and fuel cells. mrs elliott Difference Between Galvanic And Electrolytic Cell Web what's the difference between a galvanic cell and an electrolytic cell? A galvanic cell converts chemical energy to electrical. Web galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. Web there are two types of electrochemical cells: Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the.. Difference Between Galvanic And Electrolytic Cell.
From www.youtube.com
The differences between the voltaic cell and the electrolytic cell Difference Between Galvanic And Electrolytic Cell Web galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web this article explains the key differences between galvanic cell and electrolytic cell on the basis of energy conversion, redox. The main differences are outlined below: Web what's. Difference Between Galvanic And Electrolytic Cell.
From wirelibrarybrahmins.z22.web.core.windows.net
Diagram Of An Electrolytic Cell Difference Between Galvanic And Electrolytic Cell Web there are two types of electrochemical cells: Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Web galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. Web however, there are also striking differences between the two cells. A galvanic cell converts chemical energy to. Difference Between Galvanic And Electrolytic Cell.
From mavink.com
Voltaic Cells And Electrolytic Cells Difference Between Galvanic And Electrolytic Cell A galvanic cell converts chemical energy to electrical. Web there are two types of electrochemical cells: Web what's the difference between a galvanic cell and an electrolytic cell? Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. Web however, there are also striking differences between the two cells. Web a galvanic (voltaic) cell. Difference Between Galvanic And Electrolytic Cell.
From www.numerade.com
SOLVED 2 Please discuss similarities and differences between galvanic Difference Between Galvanic And Electrolytic Cell Web a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas. Web a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. The main differences are outlined below: A galvanic cell converts chemical energy to electrical. Web what's the difference between a galvanic cell and an electrolytic cell?. Difference Between Galvanic And Electrolytic Cell.